Elevate Medication Safety in Africa: Empower Your Team with Afrisummit’s Pharmacovigilance Training
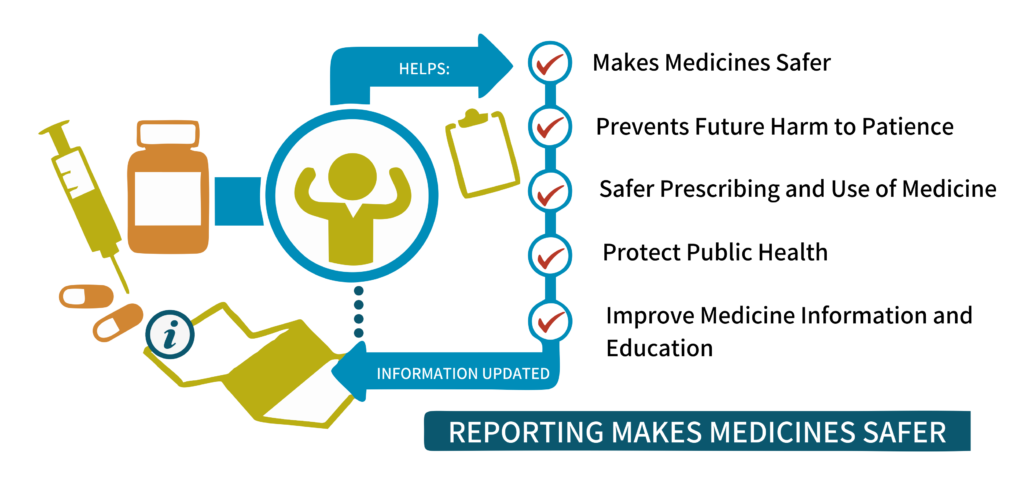
Unlock the potential of pharmacovigilance to safeguard public health across Africa. Afrisummit invites senior professionals to immerse themselves in our comprehensive training program, meticulously crafted to fortify your expertise in pharmacovigilance, adverse reaction reporting, and regulatory compliance.
Led by distinguished pharmacovigilance luminaries hailing from Africa, the Middle East, and Europe, our summit promises unparalleled insights and strategies to overcome daily challenges in medication safety.
Our dynamic curriculum focuses on fostering a robust pharmacovigilance ecosystem, fostering a culture of proactive feedback, and fostering airtight regulatory adherence. Together, we will forge a path towards a safer Africa, one meticulously managed adverse reaction at a time.
Designed exclusively for seasoned pharmacovigilance professionals, this intensive training equips you with the tools and knowledge to optimize your daily practice. By delving into regulatory intricacies and real-world implications, you’ll emerge primed to navigate the evolving landscape of medication safety with confidence.
Don’t miss this unparalleled opportunity to connect with industry visionaries, exchange invaluable insights, and chart a course towards excellence in pharmacovigilance. Join us as we unite continents to create a safer, healthier tomorrow for Africa and beyond.